Some see a gearhead. We see the nearly We see the nearly 60 joints performing every tune up
ELOCTATE Prophylaxis Offers Bleed Protection* You Can Count On.

BLEED AND JOINT BLEED PROTECTION* YOU CAN COUNT ON
Preventing bleeds and joint bleeds allows you to get back to all the things you love to do.
(SELECT ONE)
(12 years and older)
(under 12 years old)
Facts About Bleed Protection
In clinical studies of adults and adolescents using an individualized prophylaxis regimen†:



*ELOCTATE has been proven to help patients prevent bleeding episodes using a prophylaxis regimen.
†In the A-LONG study, 164 adults and adolescents ages 12-65 received ELOCTATE either every 3 to 5 days, once weekly, or on demand.
‡The ASPIRE extension study included 150 people who completed A-LONG. Data from patients treating prophylactically with ELOCTATE for at least 12 months, who had target joints at enrollment in ASPIRE. 234 out of 235 target joints were resolved. A target joint is defined as a major joint with 3 or more bleeding episodes in a consecutive 6-month period. Target joint resolution is defined as 2 or fewer spontaneous bleeds in a 12-month period.
Facts About Bleed Protection
In clinical studies of patients using an individualized prophylaxis regimen†:




*ELOCTATE has been proven to help patients prevent bleeding episodes using a prophylaxis regimen.
†In the Kids A-LONG study, 69 children ages 1-11 received ELOCTATE twice weekly. The ASPIRE extension study included 61 children who completed Kids A-LONG.
‡Data from patients treating prophylactically with ELOCTATE for at least 12 months, who had target joints at enrollment in ASPIRE. 9 out of 9 target joints were resolved. A target joint is defined as a major joint with 3 or more bleeding episodes in a consecutive 6-month period. Target joint resolution is defined as 2 or fewer spontaneous bleeds in a 12-month period.
GET RELIEF. GET CONTROL.
In a clinical study of adults and adolescents treating on demand:


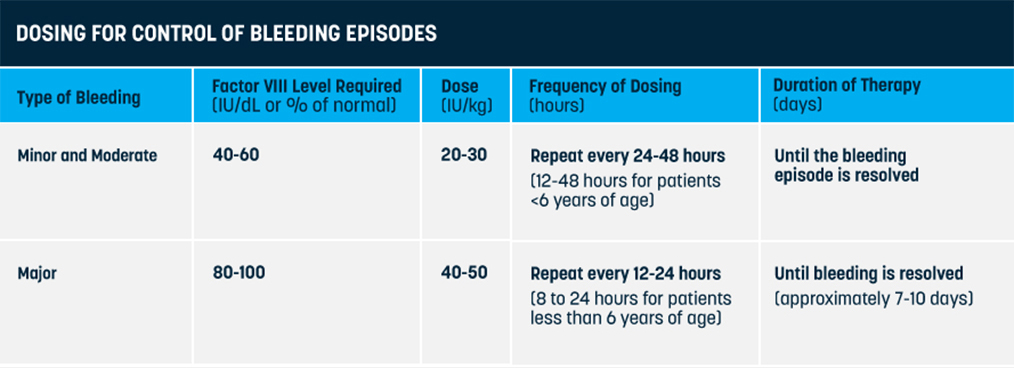
On-demand treatment and control of bleed episodes
+
GET RELIEF. GET CONTROL.
In a clinical study of patients treating on-demand:


On-demand treatment and control of bleed episodes
+

I'm happy with the bleed and joint bleed protection I'm getting from my ELOCTATE prophy. And that's important to me.
- Kenny, an ELOCTATE PEER
Note: This is a personal account of an ELOCTATE Peer™. Please talk to your healthcare provider about whether ELOCTATE may be right for you. Individual results may vary. ELOCTATE Peers have been compensated for sharing their stories.
Dosing That Lets You Do You
In clinical trials, 98.8% of patients were able to dose less frequently with ELOCTATE than with their prior standard half-life treatment.
With a variety of vial strength options, ELOCTATE offers individualized dosing and the potential for fewer infusions using a prophylaxis regimen.


See How We Stack Up
In clinical studies, ELOCTATE was shown to last 50% longer in the body than ADVATE®. Learn how extended half-life dosing made a difference for DJ.
ADVATE® [Antihemophilic Factor (Recombinant)] is a registered trademark of Baxalta Incorporated, a Takeda company.

Fc Fusion and You
Fc Fusion utilizes naturally occurring Fc receptors in your body to keep Factor VIII temporarily recirculating in your bloodstream.2-6 ELOCTATE is Factor VIII fused with an Fc protein. For the scientist in you, check out this video with a little more detail about how Fc Fusion works.
ELOCTATE is an injectable medicine that is used to help control and prevent bleeding in people with hemophilia a (congenital Factor VIII deficiency). Your healthcare provider may give you ELOCTATE when you have surgery.
Do not use ELOCTATE if you have had an allergic reaction to it in the past. Please see additional important safety information at the end of this video and full prescribing information at the link above.
The body's response to bleeding is a tightly regulated process which involves many essential clotting factors, one of which is Factor VIII. Factor VIII exists in both a turned on and turned off form and can switch between these two states.
Low levels of Factor VIII, as seen in patients with Hemophilia A, result in bleeding. The standard of care for people with severe hemophilia a involves the injection of Factor VIII. However just like Factor VIII, which is produced in the body, Factor VIII treatments breakdown overtime.
DNA is the building block that codes all our genetic information. Our scientists were able to put together the genetic code for Factor VIII, which helps with clotting and Fc a protein naturally found within your body which helps extend the amount of time proteins can circulate in the body to create a fusion. The fusion of these two proteins generates ELOCTATE, which harnesses a naturally occurring pathway to maintain Factor VIII in the bloodstream for longer.
Here's how it works. As ELOCTATE is transported in the bloodstream, the Fc portion of ELOCTATE binds with an FC receptor which exists naturally within your body. Once ELOCTATE is bound, the FC receptors are able to redirect it back toward the bloodstream, temporarily protecting it from degradation within the cell. Using the natural Fc process, ELOCTATE is able to recirculate in the body longer. Eventually, ELOCTATE is broken down, but without Fc fusion it wouldn't be able to recirculate in the bloodstream to extend the time you're protected from bleeds.
The recommended starting regimen is 50 IU per kilogram every four days as directed by your doctor. In children under six years of age the recommended starting regimen is 50 IU per kilogram administered twice weekly. The regimen can be adjusted based on your body's individual response.
IMPORTANT SAFETY INFORMATION
Do not use ELOCTATE if you have had an allergic reaction to it in the past.
Tell your healthcare provider if you have or have had any medical problems, take any medicines, including prescription and non-prescription medicines, supplements, or herbal medicines, have any allergies, are breastfeeding, are pregnant or planning to become pregnant, or have been told you have inhibitors (antibodies) to Factor VII.
Allergic reactions may occur with ELOCTATE. Call your healthcare provider or get emergency treatment right away if you have any of the following symptoms: difficulty breathing, chest tightness, swelling of the face, rash, or hives.
Your body can also make antibodies called “inhibitors” against ELOCTATE ,which may stop ELOCTATE from working properly.
Additional common side effects of ELOCTATE are headache, rash, joint pain, muscle pain and general discomfort.
If you have risk factors for developing abnormal blood clots in your body, such as an indwelling venous catheter, treatment with Factor VIII may increase this risk.
These are not all the possible side effects of ELOCTATE. Talk to your healthcare provider right away about any side effect that bothers you or that does not go away, or if bleeding is not controlled after using ELOCTATE.
INDICATION
ELOCTATE® [Antihemophilic Factor (Recombinant), FC Fusion Protein] is an injectable medicine that is used to help control and prevent bleeding in people with Hemophilia A (congenital Factor VIII deficiency). Your health care provider may give you ELOCTATE when you have surgery.


![ELOCTATE® (Antihemophilic Factor Recombinant), Fc Fusion Protein]](/Content/images/eloctate-logo.svg)