RECONSTITUTION
When infusing ELOCTATE, refer to this reconstitution video
Please see full Instructions for Use.Reconstitution and administration of ELOCTATE.
ELOCTATE® [Antihemophilic Factor (Recombinant), Fc Fusion Protein] ELOCTATE is an injectable medicine that is used to help control and prevent bleeding in people with Hemophilia A. Hemophilia A is also called congenital Factor VIII deficiency. Your healthcare provider may give you ELOCTATE when you have surgery.
Selected Important Safety Information.
Do not use ELOCTATE if you have had an allergic reaction to it in the past. Tell your healthcare provider if you have or have had any medical problems, take any medicines, including prescription and non-prescription medicines, supplements, or herbal medicines, have any allergies, are breastfeeding, are pregnant or planning to become pregnant, or have been told you have inhibitors (antibodies) to Factor VIII. Please see the end of this video for additional Important Safety Information.
Introduction.
This video will take you through the process of reconstituting and administering ELOCTATE. Remember to always refer to the instructions for use provided within the Prescribing Information. If you have additional questions, talk to your doctor. If it is your first time infusing, be sure to work with your healthcare provider or caregiver to guide you through the process. Before we get going, make sure you're working on a surface that is flat and clean, your hands are washed with soap and water, and all of your supplies are at the ready. Your kit contains a vial containing the powdered drug, packaged vial adapter, a pre-filled diluent syringe, and a plunger rod. You'll also need some alcohol wipes, infusion set tubing, and a tourniquet, or an armband to help with infusion. If you'll need more than one vial for your full dose, which is called “pooling,” you'll need a larger syringe, called a “luer lock,” which should be prescribed by your physician. Check the expiration date on the ELOCTATE kit. Do not use the product if past the expiration date. Allow the ELOCTATE vial and the diluent to come to room temperature. Do not use external heat sources such as putting the vial and/or diluent in hot water.
Reconstitution.
We'll begin with reconstitution or prepping your factor. Take care to ensure you're working with clean hands in a sanitary space. First, remove the plastic cap from the ELOCTATE vial. Then, wipe the rubber stopper of the vial with an alcohol wipe and allow it to dry. After cleaning, do not touch the rubber stopper with your hand or allow it to touch any surface. Next, completely remove the backing from the vial adapter by peeling off the lid. Do not remove the vial adapter from the package or touch the inside of the vial adapter. Keep the vial on a flat surface. Hold the vial adapter package with one hand and using the other hand, place the vial adapter over the vial. The spike should be placed directly above the center of the rubber stopper. Push the vial adapter straight down until the adapter spike punctures the center of the vial stopper and is fully inserted. After connecting the vial adapter to the vial, lift the package cover away from the vial adapter, and discard the cover. Now, take your plunger rod and, holding it by the circular disk, place the tip into the end of the syringe. Turn in a clockwise motion until it is securely attached. Once the plunger rod is attached, use one hand to hold the diluent syringe by the ridged section, right under the cap. The cap should be pointed directly upwards. With your other hand, grasp the cap and bend it at a 90° angle until it snaps off and you see the glass tip of the syringe. Do not touch the glass tip of the syringe or inside of the cap. Be sure the vial is sitting on a flat surface. Next, insert the tip of the syringe into the adapter opening on the vial. Turn the syringe in a clockwise motion until it is securely attached to the adapter. Now we can begin the reconstitution, which is the process of adding the diluent to the dry ingredient to make it a liquid. Slowly depress the plunger rod to inject all of the diluent into the vial. The plunger rod may rise slightly after this process. This is normal. With the syringe still connected to the adapter, gently swirl the vial until the product is completely dissolved. The appearance of the solution should be clear to slightly opalescent and colorless. Do not shake. Do not use the reconstituted ELOCTATE if it contains visible particles or is cloudy. If you only need a single vial to achieve your prescribed dose, let's continue. However, if you are using more than one vial, this is called pooling. Stop here, and proceed to the pooling instructions within this video. Now that the ELOCTATE is reconstituted, make sure the plunger rod is completely depressed. Turn the vial upside-down. Slowly pull on the plunger rod to draw the solution into the syringe. Be careful not to pull the plunger rod completely out of the syringe.
Next, gently unscrew the syringe from the vial adapter and dispose of the vial with the adapter still attached. Once reconstituted, ELOCTATE should be administered as soon as possible. ELOCTATE should be administered within three hours after reconstitution. Reconstituted ELOCTATE must be kept away from direct sunlight and cannot be refrigerated.
Pooling
You may need to use more than one vial to complete your dose, which is called pooling. Pooling is the process of combining two or more reconstituted vials into a larger syringe (not into the diluent syringe) prior to intravenous administration. If you are using two or more vials, follow these pooling steps. Be sure to leave the vial adapter attached to the vial, as you will need it for attaching a large luer lock syringe. Do not detach the diluent syringe or the large luer syringe until you are ready to attach the large luer lock syringe to the next vial (with vial adapter attached). Once you've reconstituted your ELOCTATE vial, remove the diluent syringe from the vial adapter by turning it counterclockwise until it is completely detached. Next, you're going to attach a separate large luer lock syringe by turning it clockwise until it is securely in place. Now that the large luer lock syringe is attached to the vial, turn the vial upside-down and, slowly pull on the plunger rod to draw the solution into the syringe. For every vial you use, you'll need to repeat the same reconstitution process for each vial and draw the solution into the larger syringe. Once you have pooled the required dose, proceed to administration using the large luer lock syringe.
Administration
ELOCTATE is administered by intravenous infusion after reconstitution of the drug powder with the diluent. Your healthcare provider should teach you how to infuse ELOCTATE. Once you have been taught to self-infuse, you can follow the instructions for use and this video. Do not administer reconstituted ELOCTATE if it contains particulate matter, is discolored, or is cloudy. Now that we've got reconstituted ELOCTATE, let's get ready to administer it through intravenous infusion. Start by attaching the syringe to the connector end of infusion set tubing by turning it clockwise until it is securely attached. Next, you'll need to find a vein for infusion, and a tourniquet helps with that by making veins larger and easier to locate. The tourniquet should be applied just above where you'll be infusing. Once your tourniquet is on, use an alcohol wipe to clean the area of skin where you will perform the infusion. Depress the plunger until all air is removed from the syringe and ELOCTATE has reached the end of the infusion set tubing. Do not push ELOCTATE through the needle. Remove the protective needle cover from the infusion set tubing. Insert the needle on the infusion set tubing into the vein. Remove the tourniquet. Always verify proper needle placement when performing intravenous administration. Slowly depress the plunger on the syringe to administer ELOCTATE. ELOCTATE should be injected intravenously over several minutes. The rate of administration should be determined by your comfort level. The small amount of drug product left in the infusion set will not affect treatment. After infusing ELOCTATE, remove the infusion set, and use a sterile gauze to put pressure on the infusion site for several minutes. Apply an adhesive bandage if necessary. Dispose of all unused solution, empty vials, and other used medical supplies in an appropriate medical waste container. And there you have it—you're one step closer to becoming an ELOCTATE-infusing pro! As a reminder, always refer to the instructions for use located in the Prescribing Information within your ELOCTATE kit before you start using ELOCTATE and each time you get a refill. There may be new information. If you have additional questions, talk to your doctor.
Important Safety Information.
Do not use ELOCTATE if you have had an allergic reaction to it in the past. Tell your healthcare provider if you have or have had any medical problems, take any medicines, including prescription and non-prescription medicines, supplements, or herbal medicines, have any allergies, are breastfeeding, are pregnant or planning to become pregnant, or have been told you have inhibitors (antibodies) to Factor VIII. Allergic reactions may occur with ELOCTATE. Call your healthcare provider or get emergency treatment right away if you have any of the following symptoms: difficulty breathing, chest tightness, swelling of the face, rash, or hives. Your body can also make antibodies called "inhibitors" against ELOCTATE, which may stop ELOCTATE from working properly. Additional common side effects of ELOCTATE are headache, rash, joint pain, muscle pain and general discomfort. If you have risk factors for developing abnormal blood clots in your body, such as an indwelling venous catheter, treatment with Factor VIII may increase this risk. These are not all the possible side effects of ELOCTATE. Talk to your healthcare provider right away about any side effect that bothers you or that does not go away, or if bleeding is not controlled after using ELOCTATE. Please see accompanying full Prescribing Information on ELOCTATE.com.
VIAL SIZE OPTIONS
The most vial strength options of any FACTOR VIII replacement therapy.
ELOCTATE is the only extended half-life therapy with 4000-IU, 5000-IU and 6000-IU dosage strengths. Having all these options means patients may be able to achieve their recommended dose with a single vial.
Talk to your doctor to see what option may be best for you. When infusing, refer to the Reconstitution video above.
*Dosing and frequency may vary based on individual patient response.
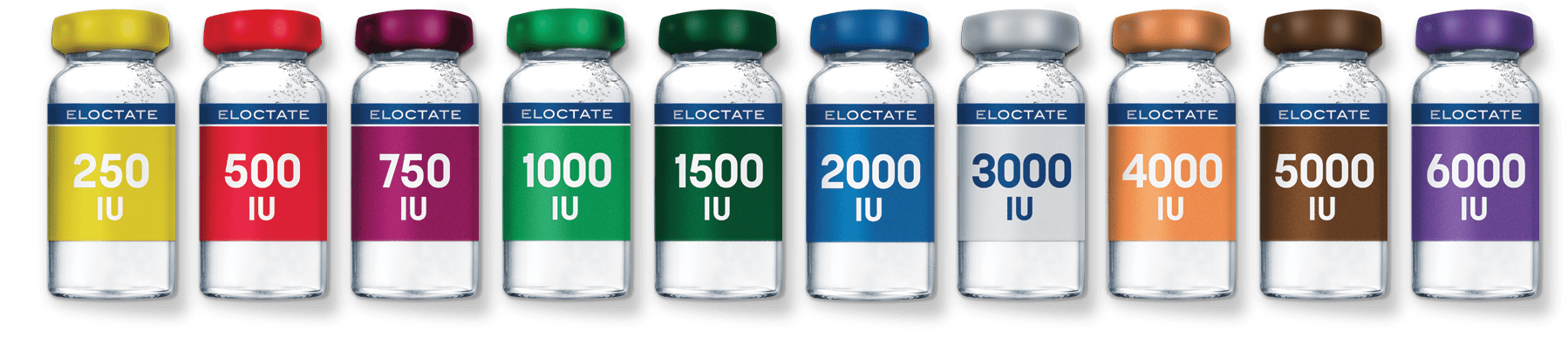
Image not representative of actual drug product vials.
CONTACT YOUR CoRe
Sanofi Hemophilia Community Relations and Education (CoRe) Managers offer education to people living with hemophilia and their families. CoRe Managers provide information about living with hemophilia and treatment options. Use our handy CoRe Locator to find the CoRe team member nearest you.
Get informed. Stay informed.
Let's stay in touch. We'll occasionally send you important information on Hemophilia A and ELOCTATE.
LET'S GET STARTEDELOCTATE® [Antihemophilic Factor (Recombinant), Fc Fusion Protein] is an injectable medicine that is used to help control and prevent bleeding in people with Hemophilia A (congenital Factor VIII deficiency). Your healthcare provider may give you ELOCTATE when you have surgery.
IMPORTANT SAFETY INFORMATION AND INDICATION
+
IMPORTANT SAFETY INFORMATION
Do not use ELOCTATE if you have had an allergic reaction to it in the past.
Tell your healthcare provider if you have or have had any medical problems, take any medicines, including prescription and non-prescription medicines, supplements, or herbal medicines, have any allergies, are breastfeeding, are pregnant or planning to become pregnant, or have been told you have inhibitors (antibodies) to Factor VIII.
Allergic reactions may occur with ELOCTATE. Call your healthcare provider or get emergency treatment right away if you have any of the following symptoms: difficulty breathing, chest tightness, swelling of the face, rash, or hives.
Your body can also make antibodies called "inhibitors" against ELOCTATE, which may stop ELOCTATE from working properly.
Additional common side effects of ELOCTATE are headache, rash, joint pain, muscle pain and general discomfort.
If you have risk factors for developing abnormal blood clots in your body, such as an indwelling venous catheter, treatment with Factor VIII may increase this risk.
These are not all the possible side effects of ELOCTATE. Talk to your healthcare provider right away about any side effect that bothers you or that does not go away, or if bleeding is not controlled after using ELOCTATE.
INDICATION
ELOCTATE® [Antihemophilic Factor (Recombinant), Fc Fusion Protein] is an injectable medicine that is used to help control and prevent bleeding in people with Hemophilia A (congenital Factor VIII deficiency). Your healthcare provider may give you ELOCTATE when you have surgery.
PLEASE SEE FULL PRESCRIBING INFORMATIONMANUFACTURED BY
Bioverativ Therapeutics Inc.
Waltham, MA 02451 USA
U.S. License #2078
CLICK HERE TO LEARN MORE ABOUT SANOFI'S COMMITMENT TO FIGHTING COUNTERFEIT DRUGS.
INDICATION
ELOCTATE® [Antihemophilic Factor (Recombinant), Fc Fusion Protein] is an injectable medicine that is used to help control and prevent bleeding in people with Hemophilia A (congenital Factor VIII deficiency). Your healthcare provider may give you ELOCTATE when you have surgery.


![ELOCTATE® (Antihemophilic Factor Recombinant), Fc Fusion Protein]](/Content/images/eloctate-logo.svg)






