Your one-stop download shop

YOU'VE GOT QUESTIONS? HERE ARE SOME ANSWERS.
If you don't see your question here, talk to your doctor or contact your Community Relations and Education (CoRe) Manager. You can locate here.
Always consult your doctor with any questions about your health or treatment.
About Hemophilia
Q
What is Hemophilia A, or Factor VIII Deficiency?
Q
How can I replace missing Factor?
Q
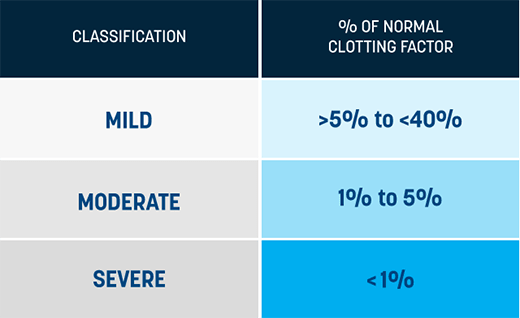
What does it mean to have a Factor level of 1% or greater?
Q
What is Fc Fusion?
About ELOCTATE
Q
What is ELOCTATE?
Q
Who should use ELOCTATE?
Q
How is ELOCTATE made?
Q
What are some of the risks with ELOCTATE?
Treating with ELOCTATE
Q
Can ELOCTATE prevent bleeds with a dosing interval of 5 days?
Q
Can ELOCTATE control bleeds when they occur?
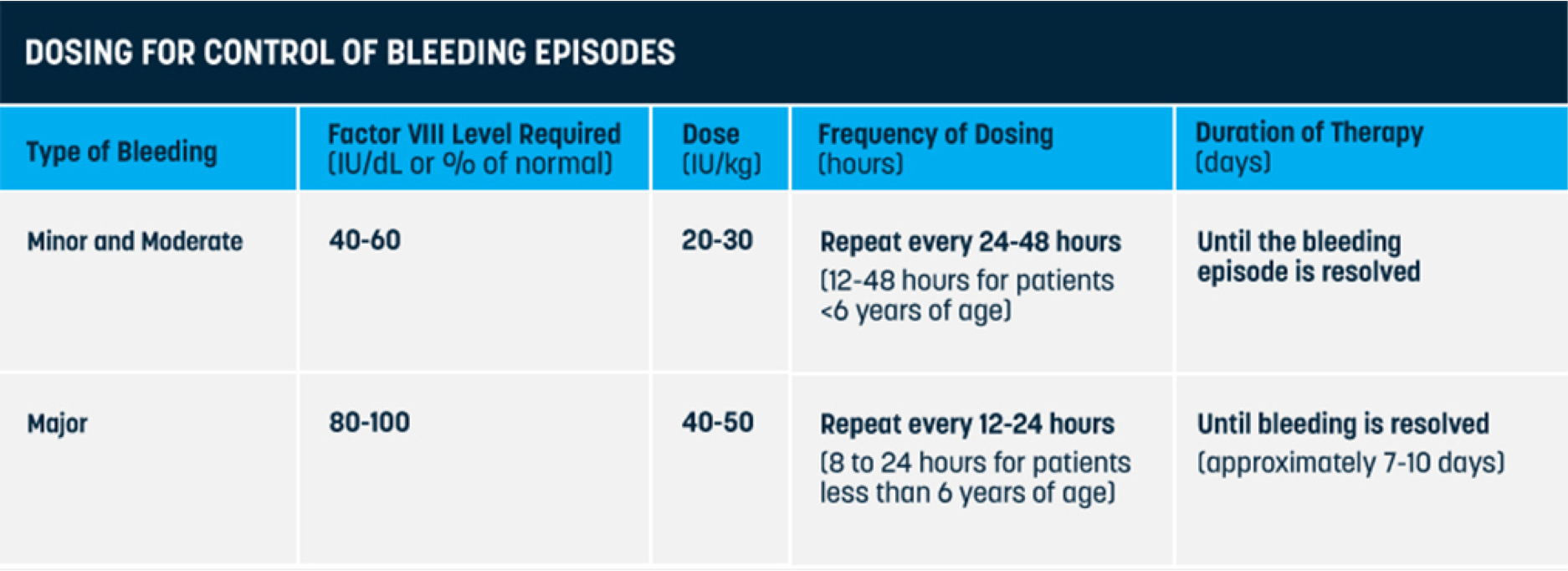
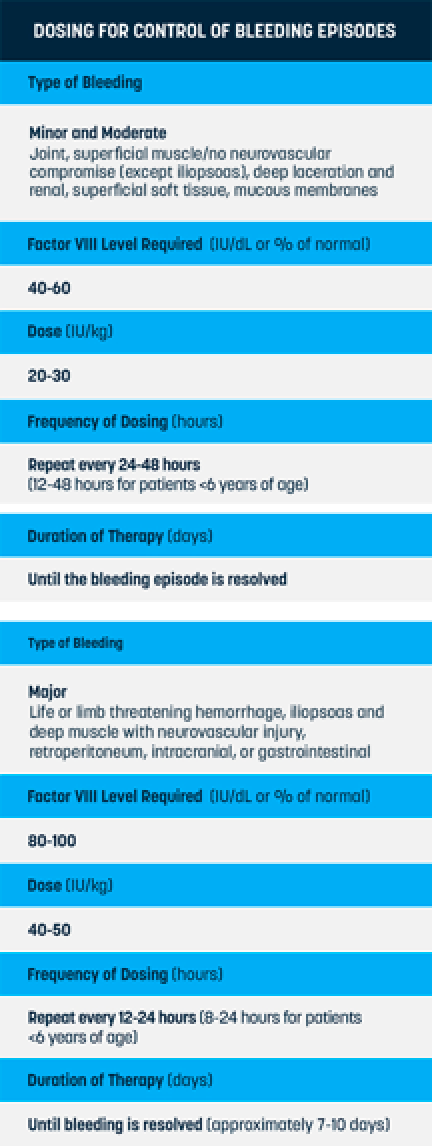
Q
What dosing regimens does ELOCTATE offer to treat bleeds?
Q
What are important questions to ask my healthcare provider about transitioning to ELOCTATE?
Q
What vial sizes are available?
About ELOCTATE Study Design
Q
How was the study designed?
Q
What is meant by the term “Previously Treated Patients” (PTPs) in
the A-LONG study?
Q
What infusion schedules were studied with ELOCTATE?
Financial Assistance
Q
Does ELOCTATE offer a free trial program?
Q
Does ELOCTATE offer a copay program?
Q
Am I eligible for financial assistance, and what benefits might I receive?
Q
Can I continue to receive ELOCTATE if I lose my insurance?
Resources You Can Count On
ELOCTATE provides extensive resources and support you can count on whether you're considering a switch, just getting started, or a long-time ELOCTATE user.

product education
Step-by-step instructions to infuse and reconstitute safely and confidently.
instructions for use reconstitution video
reconstitution video 

experiences & expectations
Real stories from real people, helpful answers to common questions, and tools to help you talk to your doctor.
peer stories get answers
get answers  doctor
discussion guide
doctor
discussion guide 

connection
Connect to local Community Relations and Education Managers and your healthcare team.
find your core microhealth app
microhealth app 
CONTACT YOUR CoRe
Sanofi Hemophilia Community Relations and Education (CoRe) Managers offer education to people living with hemophilia and their families. CoRe Managers provide information about living with hemophilia and treatment options. Use our handy CoRe Locator to find the CoRe team member nearest you.
Get informed. Stay informed.
Let's stay in touch. We'll occasionally send you important information on Hemophilia A and ELOCTATE.
LET'S GET STARTEDELOCTATE® [Antihemophilic Factor (Recombinant), Fc Fusion Protein] is an injectable medicine that is used to help control and prevent bleeding in people with Hemophilia A (congenital Factor VIII deficiency). Your healthcare provider may give you ELOCTATE when you have surgery.
IMPORTANT SAFETY INFORMATION AND INDICATION
+
IMPORTANT SAFETY INFORMATION
Do not use ELOCTATE if you have had an allergic reaction to it in the past.
Tell your healthcare provider if you have or have had any medical problems, take any medicines, including prescription and non-prescription medicines, supplements, or herbal medicines, have any allergies, are breastfeeding, are pregnant or planning to become pregnant, or have been told you have inhibitors (antibodies) to Factor VIII.
Allergic reactions may occur with ELOCTATE. Call your healthcare provider or get emergency treatment right away if you have any of the following symptoms: difficulty breathing, chest tightness, swelling of the face, rash, or hives.
Your body can also make antibodies called "inhibitors" against ELOCTATE, which may stop ELOCTATE from working properly.
Additional common side effects of ELOCTATE are headache, rash, joint pain, muscle pain and general discomfort.
If you have risk factors for developing abnormal blood clots in your body, such as an indwelling venous catheter, treatment with Factor VIII may increase this risk.
These are not all the possible side effects of ELOCTATE. Talk to your healthcare provider right away about any side effect that bothers you or that does not go away, or if bleeding is not controlled after using ELOCTATE.
INDICATION
ELOCTATE® [Antihemophilic Factor (Recombinant), Fc Fusion Protein] is an injectable medicine that is used to help control and prevent bleeding in people with Hemophilia A (congenital Factor VIII deficiency). Your healthcare provider may give you ELOCTATE when you have surgery.
PLEASE SEE FULL PRESCRIBING INFORMATIONMANUFACTURED BY
Bioverativ Therapeutics Inc.
Waltham, MA 02451 USA
U.S. License #2078
CLICK HERE TO LEARN MORE ABOUT SANOFI'S COMMITMENT TO FIGHTING COUNTERFEIT DRUGS.
INDICATION
ELOCTATE® [Antihemophilic Factor (Recombinant), Fc Fusion Protein] is an injectable medicine that is used to help control and prevent bleeding in people with Hemophilia A (congenital Factor VIII deficiency). Your healthcare provider may give you ELOCTATE when you have surgery.


![ELOCTATE® (Antihemophilic Factor Recombinant), Fc Fusion Protein]](/Content/images/eloctate-logo.svg)